Copper (II) Sulfide (CuS) nanoparticles, as a stable p-type semiconductor with strong near-infrared (NIR) absorption, have gained attention not only as optoelectronic materials but also as a photothermal agent in biomedicine for treating malignant tumors. Here, we summarize three applications of CuS nanoparticles.
Photothermal Agent :
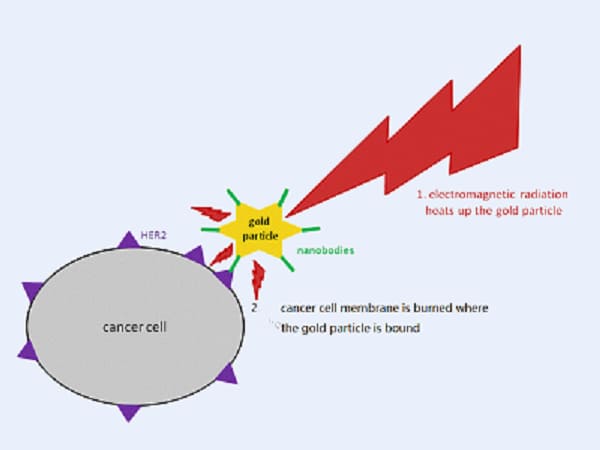
Photothermal therapy (PTT) utilizes NIR light to heat and eliminate cancer cells. CuS nano possesses excellent NIR optical properties and low toxicity, making them potential photothermal agents.
A study in ACS Nano found that hollow CuSCDs (carbon dots and CuS nano) had high photothermal efficiency. A modified version called CuSCDB@MMT7, coated with macrophage membranes and T7 peptide, specifically targeted cancer cells. The heat generated by NIR light stabilized proteins, improving tumor cell apoptosis and reducing metastasis.
Electrode materials:
CuS is a p-type semiconductor that enhances electron transfer. It disperses active substances evenly, increases active site density, and acts as a carrier. CuS is a favored electrode material due to its metallic conductivity and compatibility with polysulfide electrolytes.
Researchers published a study in CEJ where they synthesized Ni3S2 and grew CuS on it to create a Ni3S2@CuS composite. This material performed well as a positive electrode in a supercapacitor, with an energy density of 103.7 Wh/kg and a power density of 15050.3 W/kg. Even after 10,000 cycles, the Coulombic efficiency remained at 97.4%.
Photocatalysis:
CuS, with a narrow bandgap (~2 eV), can effectively capture visible light and is an excellent photocatalytic material.
Researchers enhanced CO2 photoreduction in Cu1.95S@CuS by combining defect engineering and heterojunction. This improved charge transfer, reduced recombination, and achieved 100% selectivity in converting CO2 to CO.
CrOx-CuS heterostructure decomposes water into oxygen at 10 mA cm -2, with low overpotential (190 mV vs RHE). The catalyst remains active after 36 hours of operation in alkaline media without deactivation.
CuS nano has broad applications in optics, electronics, chemical engineering, new energy, superconductors, environmental engineering, biomedicine, and other fields. However, the CuS nano industry is still in its early stages and requires continuous innovation in production and products to achieve growth.
Heeger Materials is a reputable supplier offering top-notch Copper Sulfide (CuS) Material and other Semiconductor Materials products at competitive prices, which are widely used in semiconductor industries and science fields. If you're interested, feel free to reach out to us at [email protected] for a quote, and we guarantee a response within 24 hours.


