Ferroelectric materials are pivotal in modern technology, driving innovations in electronics, sensors, and telecommunications due to their unique ability to exhibit spontaneous electric polarization that can be reversed by an external electric field. Barium Titanate (BaTiO₃) has been a cornerstone in this field, widely used for its high dielectric constant and robust ferroelectric properties. However, as technology evolves, the demand for materials with tunable properties has led to the development of Barium Strontium Titanate (BaₓSr₁₋ₓTiO₃, or BST), a material that builds on BaTiO₃’s foundation by incorporating strontium to enhance flexibility. This article explores the differences between BaTiO₃ and BST, providing insights into their composition, properties, and applications to guide material selection for specific technological needs.
At Heeger Materials Inc., we specialize in high-quality barium strontium titanate products, ensuring optimal performance for industrial and scientific applications.
Barium Titanate vs. Barium Strontium Titanate: Composition and Structure
Barium Titanate (BaTiO₃)
Barium Titanate is a ferroelectric material with the chemical formula BaTiO₃, belonging to the perovskite family with the general formula ABO₃. In this structure, barium ions (Ba²⁺) occupy the A-site, titanium ions (Ti⁴⁺) sit at the B-site, and oxygen ions (O²⁻) form an octahedral coordination around titanium. The slight displacement of the titanium ion within this octahedron creates a dipole moment, enabling ferroelectric behavior. BaTiO₃ undergoes phase transitions with temperature, shifting from a cubic (paraelectric) phase above its Curie temperature (~120°C) to a tetragonal (ferroelectric) phase below it, followed by orthorhombic and rhombohedral phases at lower temperatures.
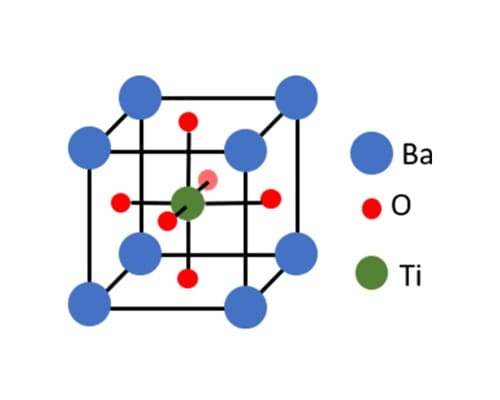
Structural Features:
- Perovskite structure with a fixed Ba:Ti:O ratio (1:1:3).
- Cubic phase at high temperatures, transitioning to tetragonal at ~120°C.
- Fixed composition limits tunability but ensures consistency.
Barium Strontium Titanate (BST)
Barium Strontium Titanate (BaₓSr₁₋ₓTiO₃) is a solid solution that substitutes strontium (Sr²⁺) for barium in the BaTiO₃ lattice. The variable ‘x’ allows the Ba:Sr ratio to be adjusted, ranging from pure BaTiO₃ (x = 1) to pure SrTiO₃ (x = 0). Strontium’s smaller ionic radius compared to barium reduces the lattice parameters, altering the phase transition temperatures and electrical properties. This compositional flexibility enables the tailoring of BST’s properties for specific applications.
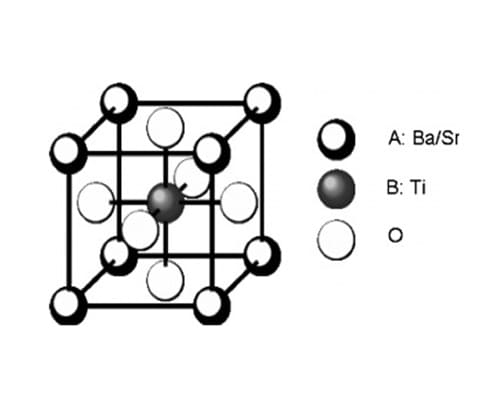
Structural Features:
- Perovskite structure with adjustable Ba:Sr ratio.
- Strontium substitution lowers the Curie temperature and modifies lattice parameters.
- Tunable composition allows customization of properties.
Comparison Table:
Property | BaTiO₃ | BST |
Chemical Formula | BaTiO₃ | BaₓSr₁₋ₓTiO₃ |
A-site Cation | Ba²⁺ only | Ba²⁺ and Sr²⁺ (variable ratio) |
Lattice Parameter | Fixed | Adjustable with Sr content |
Curie Temperature | ~120°C | Varies with Ba:Sr ratio (lower with more Sr) |
Structural Impact on Properties:
- Dielectric Constant: BST’s cubic phase → lower εᵣ (~1,000) vs. BT’s tetragonal phase (~2,000), but more tunable.
- Ferroelectricity: BT has stronger remnant polarization (~25 μC/cm²) vs. BST (~5–15 μC/cm²).
Looking for high-quality barium strontium titanate and barium titanate products? Explore Heeger Materials' selection.
Barium Titanate vs. Barium Strontium Titanate: Physical Properties
The main difference between BT and BST lies in their dielectric properties. Barium Titanate has excellent dielectric properties at room temperature but loses its ferroelectricity above 120°C due to a phase transition. Barium Strontium Titanate, with Strontium enhancing its temperature stability, can be tailored to operate at higher temperatures without phase changes. BST also generally has a higher dielectric constant than BT, particularly at low Strontium levels, but this decreases with more Strontium. Additionally, BST has better mechanical strength and wear resistance due to Strontium's influence on the crystal lattice.
Property | BaTiO₃ | BaₓSr₁₋ₓTiO₃ (x=0.7) |
Density (g/cm³) | 6.02 | 5.85 |
Thermal Conductivity | 3.5 W/mK | 2.8 W/mK |
CTE (ppm/K) | 10.4 | 9.2 |
Band Gap (eV) | 3.2 | 3.3 |
Barium Titanate vs. Barium Strontium Titanate: Electrical Properties
BT and BST's electrical properties are key to their application suitability. Barium Titanate offers a high dielectric constant but is sensitive to temperature fluctuations. Barium Strontium Titanate, with Strontium, maintains stable performance across a broader temperature range. Tuning Strontium content optimizes BST's dielectric properties, enhancing capacitance and reducing loss. Additionally, BST has higher dielectric breakdown strength and lower leakage currents, making it ideal for high-power, high-frequency applications.
1. Dielectric Behavior Comparison
Parameter | BaTiO₃ | Ba₀.₇Sr₀.₃TiO₃ | Implications |
Dielectric Constant (εᵣ) | 1,500–2,000 (RT) | 800–1,200 (RT) | BST offers better frequency stability |
Curie Temperature (T꜀) | 120°C (sharp transition) | Adjustable (-50°C to 50°C) | BST enables tunable applications |
Tunability (%) | <5% @ 40V/μm | 50–70% @ 40V/μm | BST preferred for RF devices |
Loss Tangent (tan δ) | 0.01 @ 1kHz | 0.002–0.01 @ 10GHz | BST is superior for high-frequency use |
Temperature Stability | ±15% (25–100°C) | ±5% (25–100°C) | BST is more reliable in varying temperatures |
2. Ferroelectric Properties Comparison
Property | BaTiO₃ | Ba₀.₇Sr₀.₃TiO₃ | Functional Impact |
Remnant Polarization (Pᵣ) | 20–25 μC/cm² | 5–15 μC/cm² | BaTiO₃ is better for FeRAM |
Coercive Field (E꜀) | 1.2–1.8 kV/mm | 0.7–1.2 kV/mm | BST lowers switching energy |
Polarization Switching Time | 10–100 ns | 1–10 ns | BST is faster for neuromorphic devices |
Fatigue Resistance | 10⁸ cycles | >10¹⁰ cycles | BST is more durable for memory |
Hysteresis Loop Shape | Square | Slimmer | BST is better for analog memristors |
3. Conductivity & Leakage Comparison
Characteristic | BaTiO₃ | Ba₀.₇Sr₀.₃TiO₃ | Optimization Strategy |
DC Leakage (A/cm²) | 10⁻⁷ @ 100kV/cm | 10⁻⁸–10⁻⁹ (Mn-doped) | Doping is critical for BST reliability |
Breakdown Strength | 100–300 kV/cm | 300–500 kV/cm | BST handles higher fields |
Oxygen Vacancy Concentration | High (10¹⁸ cm⁻³) | Medium (10¹⁷ cm⁻³) | BST is more resistant to degradation |
Ionic Conductivity | 10⁻⁹ S/cm @ 300K | 10⁻¹⁰ S/cm @ 300K | BST is better for capacitor insulation |
Electrode Interface | Prone to Schottky barriers | Ohmic contact achievable | BST is easier to integrate with metals |
Barium Titanate vs. Barium Strontium Titanate: Applications
BT offers a high dielectric constant and strong piezoelectric properties, ideal for capacitors, sensors, and actuators, but its temperature sensitivity limits its use in temperature-sensitive applications. BST, with Strontium, improves temperature stability and mechanical strength, making it better for high-frequency and high-power applications. However, its dielectric constant decreases with higher Strontium content, limiting its use in high-capacitance applications. Additionally, manufacturing BST with precise Strontium control can be challenging for large-scale production.
1. Capacitor Technologies
Application | BaTiO₃ (BT) | BaSrTiO₃ (BST) | Material Advantage |
MLCCs | High-εᵣ X7R/X5R ceramics (εᵣ~2,000) | Tunable RF capacitors (εᵣ~1,200) | BT: Higher capacitance density |
Decoupling Caps | Consumer electronics | 5G mmWave ICs | BST: Lower loss @ GHz frequencies |
Energy Storage | High-voltage capacitors | Fast-discharge pulse capacitors | BT: Higher breakdown strength |
2. Memory Devices
Type | BT Usage | BST Usage | Key Differentiator |
FeRAM | Commercial (Fujitsu, TI) | Emerging prototypes | BT: Higher Pᵣ (25 vs. 15 μC/cm²) |
DRAM | Not used | Deep-trench capacitors | BST: Scalable to <10nm nodes |
Memristors | Binary switching | Analog synaptic devices | BST: Better linear conductance tuning |
3. RF/Microwave Systems
Component | BT Performance | BST Performance | Superior Material |
Phase Shifters | Not applicable | 60GHz, 75% size reduction | BST: Field-tunable εᵣ |
Filters | Fixed-frequency | Reconfigurable (1-40GHz) | BST: 50-70% tunability |
Antennas | Not used | Beam-steering metasurfaces | BST: Zero-power tuning capability |
4. Emerging Applications
Field | BT Implementation | BST Implementation | Rationale |
Neuromorphic | Limited | Crossbar arrays (20 TOPS/W) | BST: Analog resistance switching |
Quantum | Not applicable | Qubit couplers (Q>10⁶ @4K) | BST: Cryogenic stability |
Flexible Electronics | Thick-film sensors | Thin-film transistors (<100nm) | BST: Low-temp processing advantage |
5. Industrial & Energy
Use Case | BT Solution | BST Solution | Technical Edge |
Piezoelectric | Actuators (d₃₃~190pC/N) | Low-strain tunable transducers | BT: Stronger piezoelectric response |
Thermistors | PTC thermistors (Tc~120°C) | Broad-range sensors (Tc adjustable) | BST: Customizable Tc |
PV Systems | Not used | Ferroelectric photovoltaics | BST: Enhanced carrier separation |
Request a custom quote for high-quality barium strontium titanate and barium titanate.
Barium Titanate vs. Barium Strontium Titanate: Synthesis and Processing
BaTiO₃ is typically synthesized through solid-state reactions, where barium carbonate (BaCO₃) and titanium dioxide (TiO₂) are heated at 1100–1400°C, with alternatives like sol-gel or hydrothermal methods offering better control over particle size and purity for large-scale production. In contrast, BST synthesis is more complex due to the need to precisely control the Ba:Sr ratio. Solid-state reactions require accurate mixing of BaCO₃, SrCO₃, and TiO₂, while sol-gel methods improve homogeneity. For microwave devices, thin-film BST is often produced via pulsed laser deposition or chemical vapor deposition, where processing conditions play a critical role in the material's properties.
1. Synthesis Methods
1.1 Solid-State Reaction (Conventional Method)
Parameter | BaTiO₃ (BT) | BaₓSr₁₋ₓTiO₃ (BST) | Key Differences |
Precursors | BaCO₃ + TiO₂ | BaCO₃ + SrCO₃ + TiO₂ | BST requires a Sr source |
Calcination Temp. | 1100–1300°C | 1200–1400°C | BST needs a higher temperature for Sr incorporation |
Phase Purity | Single-phase (tetragonal) | Requires careful stoichiometry control | BST is more sensitive to the Sr/Ba ratio |
Particle Size | 0.5–5 µm (agglomerated) | 0.2–3 µm (finer due to Sr doping) | BST tends to form smaller grains |
1.2 Sol-Gel Process (Chemical Solution Deposition)
Parameter | BT | BST | Key Differences |
Precursors | Ba-alkoxide, Ti-alkoxide | Ba/Sr-alkoxide, Ti-alkoxide | BST requires the Sr precursor |
Annealing Temp. | 600–800°C | 700–900°C | BST needs a higher crystallization temperature |
Film Quality | Dense, crack-free | More prone to cracking (Sr volatility) | BST films require slower drying |
Thickness Range | 50–500 nm | 30–400 nm | BST is harder to deposit uniformly |
1.3 Hydrothermal/Solvothermal Synthesis
Parameter | BT | BST | Key Differences |
Reaction Temp. | 150–250°C | 180–300°C | BST requires higher pressure/temperature |
Particle Size | 20–100 nm | 10–80 nm | BST yields finer nanoparticles |
Morphology | Cubic/tetragonal | Spherical/rod-like | BST shape depends on the Sr content |
Yield | High (>90%) | Moderate (70–85%) | Sr incorporation reduces yield |
2. Thin-Film Deposition Techniques
2.1 Sputtering (PVD)
Parameter | BT | BST | Key Differences |
Target Composition | BaTiO₃ ceramic | BaₓSr₁₋ₓTiO₃ | BST targets are harder to sinter |
Substrate Temp. | 400–600°C | 500–700°C | BST requires a higher temperature for crystallinity |
Film Stress | Moderate (compressive) | High (tensile) | BST is more prone to cracking |
Deposition Rate | 5–10 nm/min | 3–8 nm/min | BST is slower due to the Sr volatility |
2.2 Pulsed Laser Deposition (PLD)
Parameter | BT | BST | Key Differences |
Laser Energy | 2–3 J/cm² | 2.5–4 J/cm² | BST needs higher energy for Sr ablation |
Oxygen Pressure | 0.1–0.3 mbar | 0.2–0.5 mbar | BST requires more O₂ for stoichiometry |
Epitaxial Growth | Common on SrTiO₃ | Challenging on Si | BST lattice mismatch issues |
Film Quality | High crystallinity | More defects (Sr segregation) | BST is harder to optimize |
2.3 Atomic Layer Deposition (ALD)
Parameter | BT | BST | Key Differences |
Precursors | Ba(TMHD)₂, Ti(OiPr)₄ | Ba/Sr(TMHD)₂, Ti(OiPr)₄ | BST needs a Sr precursor |
Growth Temp. | 250–350°C | 300–400°C | BST requires a higher temperature |
Conformality | Excellent (high aspect ratio) | Slightly lower (Sr diffusion) | BST more complex |
Thickness Control | ±1 nm | ±2 nm | BST less precise |
3. Processing Challenges
Issue | BT | BST | Solution |
Oxygen Vacancies | Moderate | Severe | Mn/Mg doping (BST) |
Cracking | Rare | Common | Slower annealing (BST) |
Stoichiometry Control | Easy | Difficult | Combinatorial synthesis (BST) |
Interface Reactions | Minimal | Severe (with Si) | Buffer layers (BST) |
4. Summary of Best Methods by Application
Application | Preferred BT Synthesis | Preferred BST Synthesis |
MLCCs | Solid-state reaction | Sol-gel + doping |
Thin-Film Caps | Sputtering | ALD/PLD |
FeRAM | Sol-gel | PLD (epitaxial) |
RF Tunable Devices | Not used | Sputtering/ALD |
At Heeger Materials, we supply high-performance barium strontium titanate products in various forms and specifications for multiple industrial and research applications.
Barium Titanate vs. Barium Strontium Titanate: Advantages and Limitations
Barium Titanate (BT) has a high dielectric constant and strong piezoelectric properties, making it ideal for capacitors, sensors, and actuators. However, its temperature sensitivity limits its use in temperature-sensitive applications. Barium Strontium Titanate (BST) improves temperature stability and mechanical strength, making it better for high-frequency and high-power applications. However, BST’s dielectric constant decreases with more Strontium, which limits its use in high-capacitance applications, and its manufacturing is more complex due to the need for precise control over the Strontium content.
1. Barium Titanate (BT) – Advantages & Limitations
✔ Advantages of Barium Titanate (BT)
Property | Performance | Applications |
High Dielectric Constant (εᵣ) | 1,500–2,000 (RT) | MLCCs, capacitors |
Strong Ferroelectricity | Pr ≈ 25 μC/cm², high Tc (120°C) | FeRAM, piezoelectric actuators |
Thermal Stability | Stable up to 120°C (Tc) | PTC thermistors, sensors |
Low Cost | $50–100/kg (mass-produced) | Consumer electronics, ceramics |
Ease of Processing | Simple solid-state synthesis | Bulk ceramics, thick films |
❌ Limitations of Barium Titanate (BT)
Challenge | Impact | Mitigation Strategies |
Temperature Sensitivity | Sharp εᵣ drop at Tc | Dopants (Ca, Zr) to broaden Tc |
High Loss at RF Frequencies | tan δ > 0.01 @ GHz | Not suitable for high-frequency use |
Brittleness | Prone to cracking in thin films | Composite reinforcement |
Limited Tunability | εᵣ variation <5% with field | Not used for tunable devices |
2. Barium Strontium Titanate (BST) – Advantages & Limitations
✔ Advantages of Barium Strontium Titanate (BST)
Property | Performance | Applications |
Field-Tunable εᵣ | 50–80% tunability @ 40 V/µm | RF varactors, phase shifters |
Low High-Frequency Loss | tan δ ≈ 0.002–0.01 @ 10–100 GHz | 5G/6G antennas, microwave filters |
Adjustable Tc | Tunable (-50°C to +150°C) via Sr content | Broad-range sensors |
Scalability | Compatible with <10 nm nodes | Advanced DRAM, FeRAM |
Cryogenic Stability | Low loss (tan δ < 0.001 @ 4K) | Quantum computing resonators |
❌ Limitations of Barium Strontium Titanate (BST)
Challenge | Impact | Mitigation Strategies |
Oxygen Vacancies | High leakage current (10⁻⁶ A/cm²) | Mn/Mg doping (↓ to 10⁻⁹ A/cm²) |
Processing Complexity | Requires precise Sr/Ba ratio | Combinatorial optimization |
High Cost | $300–500/kg (vs. BT’s $50/kg) | Aqueous sol-gel methods |
Thin-Film Stress | Cracking due to Sr volatility | Buffer layers (MgO, LSAT) |
Environmental Concerns | Toxic precursors (alkoxides) | Green chemistry alternatives |
3. Direct Comparison: Barium Titanate vs. Barium Strontium Titanate
Parameter | Barium Titanate (BT) | Barium Strontium Titanate (BST) | Winner |
Dielectric Constant | Higher (εᵣ ~2,000) | Lower (εᵣ ~1,200) but tunable | BT for density |
Frequency Range | <1 GHz (high loss) | Up to THz (low loss) | BST for RF |
Ferroelectric Polarization | Stronger (25 μC/cm²) | Weaker (5–15 μC/cm²) | BT for FeRAM |
Temperature Stability | Sharp transition at Tc | Broad/adjustable transition | BST for sensors |
Cost | $50–100/kg | $300–500/kg | BT for mass production |
Scalability | Limited to below 100 nm | Compatible with <10 nm nodes | BST for advanced nodes |
BaTiO₃ and BST represent two generations of ferroelectric materials, each with unique strengths. BaTiO₃’s high dielectric constant and robust ferroelectricity make it a reliable choice for traditional applications, while BST’s tunability enables advanced applications in microwave and memory technologies. The choice between them depends on application needs, balancing cost, performance, and flexibility. BST’s potential in emerging fields like 5G and photonics highlights the importance of material innovation.
For top-quality barium strontium titanate products, Heeger Materials provides tailored solutions for various applications.
Looking for premium barium strontium titanate products? Contact us today!


